Exclusive drugs generally mean that a certain pharmaceutical company has the exclusive right to produce and sell a certain drug. It can be subdivided into exclusive active ingredients (exclusive generic name ingredients), exclusive varieties (different dosage forms for the same ingredient), and exclusive product regulations. Commonly, it can be divided into drugs Feature exclusives and product exclusives. Exclusive medicines are often perceived as having high efficacy and high prices, but is this really the case?
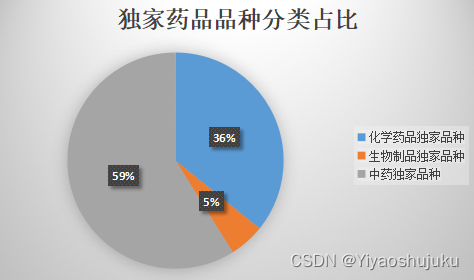
According to the statistics of the Yaorongyun medical database, the total number of exclusive drugs of various varieties currently amounts to 10,497, of which chemical drugs account for 3,759 types, biological products account for 556 types, and traditional Chinese medicines account for 6,180 types. The biggest problem in the domestic pharmaceutical market is the excess of varieties . It emerged under the influence of the supply-side reform of the pharmaceutical market, the consistency evaluation of generic drugs, the two-invoice system, and volume-based procurement. As a result, competition for clinician prescriptions has entered a white-hot stage. In this situation, more and more professionals in the industry agree that exclusive drugs do not necessarily have the characteristics of high efficacy and high price , because even for the same indication, the competition of similar drugs is ubiquitous.
How to find high-potential exclusive drug varieties?
①First of all, market demand must be studied. Look for products with huge demand but not enough supply in the market, investigate the market prospect of exclusive drug varieties, and its sales can be used as a very important reference indicator, which can find high-value exclusive drug varieties.
② Study the review information of exclusive drugs: analyze the registration review data information of exclusive drugs, track evaluation
③Research on drugs with technical advantages: drug technology is extremely valuable and has the ability to occupy the market.
④Find feasible drugs: such as patent term, production cost and clinical efficacy evaluation.
⑤Research on drugs with favorable policies: Policies play an important role in determining market trends and the direction of drug development. Policy changes will have different impacts on R&D and production companies, so exclusive drugs that conform to policy trends should be sought.
About the market sales TOP200 list of exclusive drugs and screening methods
The screening of high-sales varieties of exclusive drugs can be collected through the reports of major media, but the timeliness and expandability of such information are very poor. If you want to really conduct detailed research, it is recommended to use professional medical databases, not only It can screen and consult target exclusive drugs from multiple angles based on the actual situation of its own company, and also has the function of analyzing and predicting the trend of the drug market. For example, below, the author combines the Yaorong Cloud database, which is the most used by pharmaceutical companies, to screen and check the top 300 exclusive drug varieties in the market sales.
①First, register and log in the enterprise version of the drug Rongyun database, enter the market sales database, and screen and export the top 300 drugs with sales.
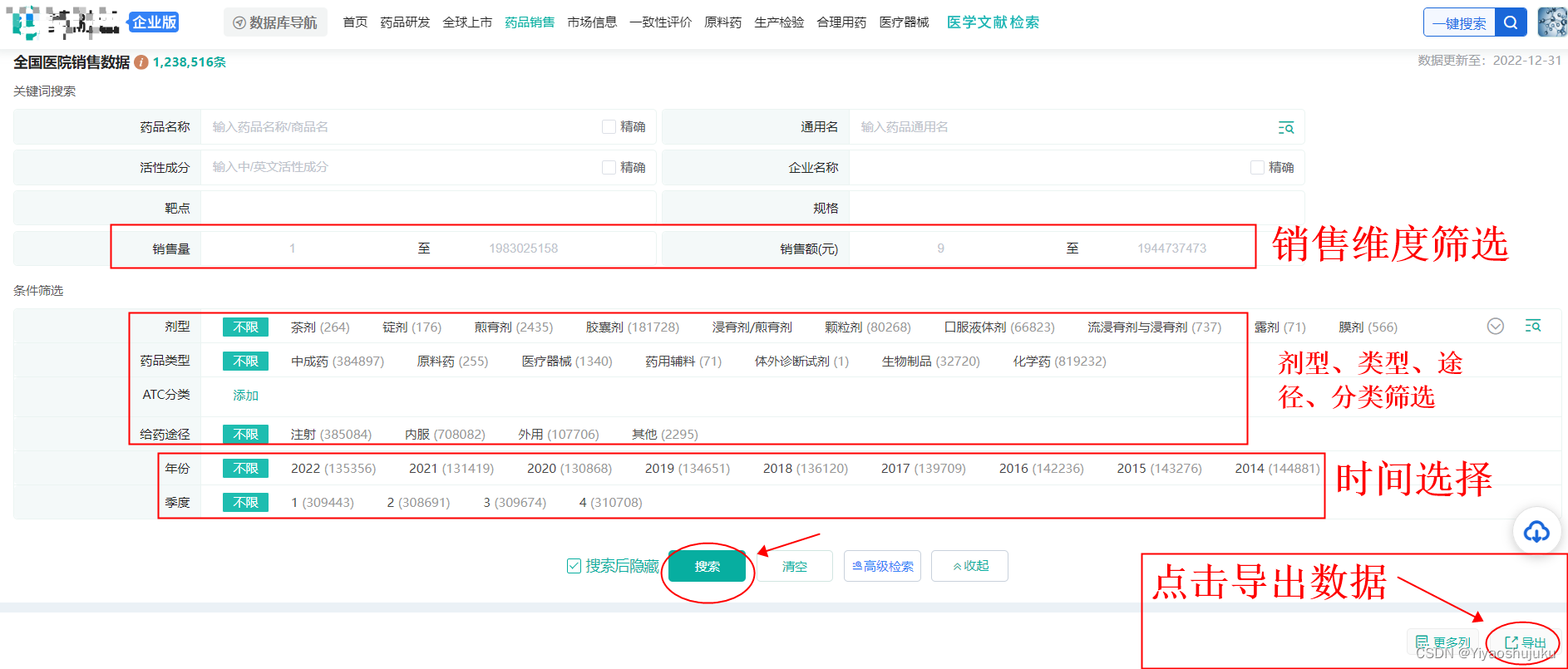
Image source: Yaorong Cloud - national hospital sales data
②Enter Yaorongyun-Global Listing-China Approval Database, click 'Advanced Search' to import the data in step ① in batches for retrieval, and then select 'Yes-Exclusive Drug' or other information from the options to filter and query, and you can quickly check Top200 exclusive medicines.

Image source: Yaorong Cloud - China Approval Database
The author screened the list of exclusive medical insurance drugs with sales of more than 1.5 billion in Yaorongyun-National Hospital Sales (full terminal) in 2021 as follows (excluding traditional Chinese medicines)
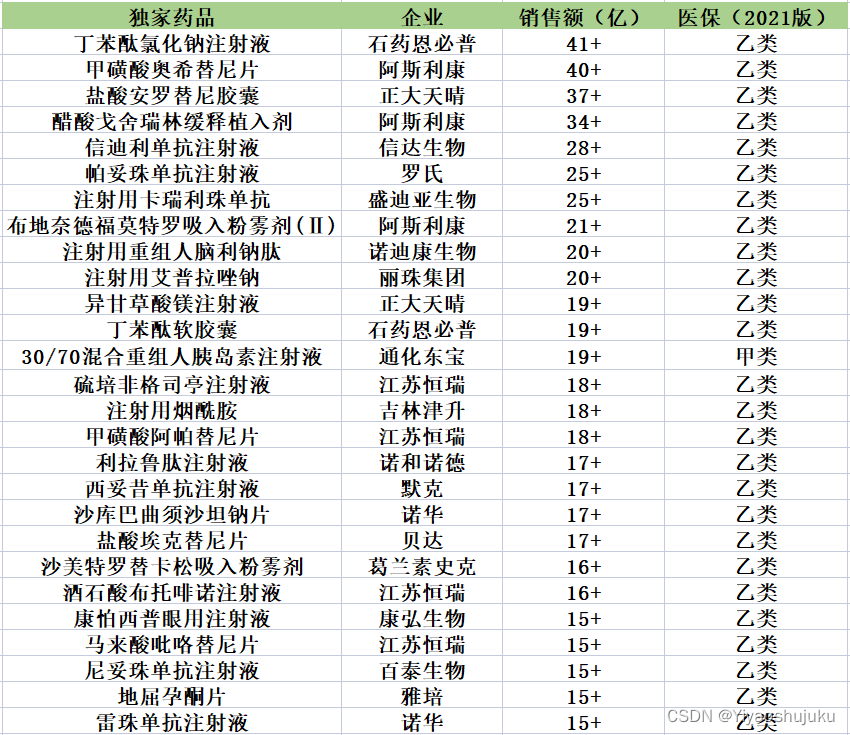
Image content data source: Yao Rongyun
Here, the author only exports part of the list and its sales. For more in-depth research, you can select and screen out the corresponding sales in 2020 and 2022 to calculate the annual growth rate of the target exclusive drug, and judge its market demand and potential.
Yaorong Cloud-China Approval Documents Database combines a series of relevant attribute information such as all domestic drug production approval information, drug treatment field classification, medical insurance drug information, national essential drug list, national non-prescription drug list, protected traditional Chinese medicine varieties, and drug company information. A valuable source of information and reference for drug approval. The specific value is reflected in the following points:
①Market information: The China Approval Document Database records the basic information of the drug, listing information, medical insurance catalog, and the research and development history of the document number, etc. This information plays an important role in understanding medicines.
② Competitive product analysis: The approval database provides comprehensive information that can help companies conduct competitive product analysis. In the process of new drug development, comparing exclusive drugs with existing drugs on the market is beneficial to determine product differentiation and competitive strategies.
③Research and development direction: the approval database provides the approval status of various drugs, which can provide reference for enterprises to develop new products. Through the query of the approval database, you can find out whether the disease treatment methods on the market meet the demand, and gain insight into the research and development direction with market value.
④ Investment decision-making: The approval database contains the research and development status of various companies, and the phased approval information of listed companies and other pharmaceutical companies in the product development process, which can provide investors with investment decision-making reference.
In addition, other screening items can be used to select special projects, major decisions, and potential new drugs, screen target drugs and sales, quickly find value depressions, and help companies efficiently search and screen high-potential exclusive drugs, which has important guiding significance for the development of the drug market .